Scene 1: A toothache had me sitting in a comfortable endodontist’s chair, surrounded by state-of-the-art dental equipment. The smell of paint and new carpeting, in addition to soothing music, was in the air, and the lighting quality was both very good and comfortable. To avoid thinking about a possible root canal, I distracted myself by listing what I thought was lighting the space without looking up. I started noting my observations in my head. CCT was warm (probably 3,000 K), very good CRI (probably 90+), high R9 (good skin tones, certainly positive, maybe by a lot), high scalloped shadows on the wall (several “smaller” fixtures?), well-blended lighting on the walls a couple of feet below the ceiling line (not CFLs, probably linear sources), very uniform light at work surface heights throughout the entire space, no intense glare sources showed in the highly reflective equipment surfaces (definitely not CFL). I could hear someone coming so I quickly wrapped up my lighting audit. I guessed the room was lit with 3,000 K T5 fluorescent lamps in quite a few good volumetric lighting fixtures, possibly 4-foot but probably shorter, and probably not HO.
Scene 2: After explaining the need for an immediate root canal and readying the appropriate tools and supplies, the soft-spoken endodontist said, and with way too much enthusiasm, “We have reached the tipping point!” as I was reclined into position. From my new vantage point, to my surprise, I could clearly see 2 by 2, two-lamp, U-lamp, T12 fluorescent lamps and fixtures. I didn’t see that coming.
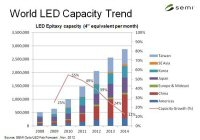
Scene 3: Prognosticators have recently said that the “LED tipping point” is behind us; therefore, why isn’t this newly-equipped, recently-renovated, state-of-the-art office lit with LEDs? Many likely think the explanation is complex, but I think it is pretty simple. Prognosticators come and go, but “lighting” evolves.
Scene 4: “Lighting technology” is mostly important to “technologists,” whereas, a reliable, cost-effective and comfortably-lit space is what is important to the customer. LEDs are a light source technology and not a lighting product or solution. LEDs can and will enable reliable, cost-effective and comfortably-lit spaces, but that won’t happen overnight. Have we forgotten the trials, tribulations and time it took to “evolve” from T12 to T8, from magnetic to electronic ballasts, or from T8 to T5?
Conclusion: Comparing that which comprised the fluorescent “evolution” of the past 30 years to the “evolution” awaiting us in/with solid state lighting is simultaneously exciting and sobering to those who understand the potential benefits, business opportunities, added functionality and challenges which might come along with solid state lighting. I look forward to the day when my house recognizes my approach and turns on my porch light to minimize my key fumbling, when sensors recognize me beginning to sit in my favorite chair and turn on my reading lamp to a high intensity/warm color, and when those same sensors recognize me dozing and dim that reading lamp to a much lower intensity. I am excited about a future where concepts like this surround us in the workplace. Although I wouldn’t classify most consumers and users as “technologists,” it is clear to me that most can be aggressive consumers of “technology” with smart phones, tablet computing devices and cars that parallel-park themselves being only a few examples. The work will be challenging, but interesting. The benefits will initially be difficult to document with numbers, but nonetheless evident. Patience (by all) will be required but exciting times and opportunities lie ahead for lighting that leads.